A prospective evaluation of the use of honey dressings to manage burn wounds
Written by: Jacky Edwards Burns Nurse Consultant, Burn Centre, Wythenshawe Hospital, Wythenshawe
The moist, dead tissue within a burn is a nutrient-rich medium that will support the growth of a variety of bacterial species, placing burns patients at greater risk of infection than those with other wound types. Traditionally, burn wounds have been managed with topical silver based dressings, but a Cochrane Review suggests that there is evidence that Honey may have a role to play. A prospective evaluation was undertaken to assess the performance of Actilite or Algivon Plus on burn wounds.
The nature of burns, which by definition contain devitalised tissues, makes these injuries more susceptible to bacterial colonisation than most other types of wound. The moist, dead tissue within the burn, alongside the surrounding damaged and oedematous tissues, provides a nutrient medium that will support the growth of a variety of bacterial species.
Several factors contribute to burn wound infection, notably, the destruction of the skin barrier, the presence of necrosis and sero-sanquinous exudate and impaired immune function (DeSanti, 2005). The risks are commensurate with the depth and extent of the burn, the health and age of the patient, local perfusion of the tissues and use of systemic antibiotics (Wounds UK, 2011).
Traditionally, burn wounds have been managed with topical silver based dressings, but a Cochrane review suggests that there is evidence that Honey may have a role to play (Jull et al, 2008). Honey has been used for over 2000 years to treat wounds, and whilst having a recent resurgence in popularity, it may still not be given its deserved recognition. The combination of Manuka honey and Manuka oil has been demonstrated in vitro to be effective against a number of major wound infecting organisms including methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci and Providencia stuartii (StephenHaynes and Callaghan, 2011).
Honey has many other properties that make it an ideal wound dressing, alongside its antiseptic properties, which reduce bacterial load, infection and odour, its high osmotic pressure promotes autolytic debridement and a moist wound healing environment (Belcher 2012).
Methods
Algivon Plus (Advancis Medical) is comprised of a calcium alginate fleece impregnated with medical-grade Manuka honey. The alginate fibres retain their structure when wet, swelling to absorb exudate and forming a soft gel that ensures a moist wound environment and prevents adhesion. Actilite (Advancis Medical) is a light viscose net dressing coated with antibacterial Manuka honey and Manuka oil. The dressing is designed to protect a wound, promote healing, and allow the passage of exudate (Advancis Medical, 2008).
This study was a prospective evaluation of the use of honey products in burns. The primary objective was to assess the performance of Actilite or Algivon Plus on burn wounds. This was assessed by patient comfort while wearing the dressing, pain during dressing changes, ease of product application and removal, product conformability, nonadherence to the wound, and the ability to manage wound exudate.
The secondary objective was to promote the education of ward staff concerning suitability of honey dressings for burn wounds and to assess long-term development of abnormal scarring.
Twenty patients were included in the evaluation, 10 received Algivon Plus dressings, and 10 Actilite dressings. Patient selection is imperative when using these products as some patients with more superficial burns report stinging. A minimum of three dressing changes were carried out for each patient. A 10-point Likert-type scale was used to assess both dressing properties (0 being least effective and 10 being most effective) and pain (0 being no pain and 10 being the most pain). All patients were followed-up 1-month post-healing to assess hypertrophic scarring. The male-to-female ratio was 3 : 1, which is normal in burn injury populations. The age range was 23–51 years with a mean age of 35.75 years.
Results
Both products performed well, although patients were happier with the Actilite dressing. The Actilite dressing was also found to be of more use in burn wounds that had broken down. The Algivon Plus dressing was found to be particularly useful for facial burns and had a greater debriding action, which was useful on some of the burns that were being conservatively treated.
Only one wound was clinically infected prior to application of the dressing. No wounds developed infection during or before the end of treatment, which is one of the key objectives when managing burn wounds.
Result of the dressing properties are shown in Figure 1. Figure 2 shows that the mean pain scores for both dressings were relatively low, with only Actilite being more painful on removal as it had often adhered and had to be soaked off.

Figure 1. Mean dressing property scores (0 being least effective and 10 being most effective).

Figure 2. Mean pain scores (0 being no pain and 10 being the most pain).
Case Study 1
Mr X is a 23-year-old man who works as a circus performer. He sustained 85% total body burns from a gas explosion in October 2012. He was initially was treated with split thickness skin grafts using the MEEK Mesher® (Humeca). The majority of areas healed by December 2012, but some areas of breakdown appeared on both arms and thighs following a S. aureus infection – one of the most commonly isolated organisms in burns (Subrahmanyam et al, 2003). He was treated with a range of products and some regrafting of areas on the upper arms was undertaken.
Mr X was commenced on Actilite on both his right upper arm and shoulder (Figure 3a) and thigh (Figure 4a). The improvement in Mr X’s right upper arm and shoulder wounds are shown at 6, 18, and 32 days following initiation of treatment with Actilite (Figures 3b–d, respectively). The improvement in Mr X’s thigh wounds are shown at 8, 15, and 21 days following initiation of treatment with Actilite (Figures 4b–d, respectively).
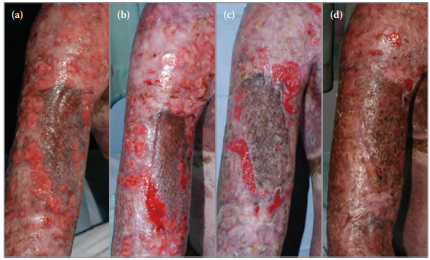
Figure 3. Mr X's upper arm and shoulder wounds during the course of treatment with Actilite.
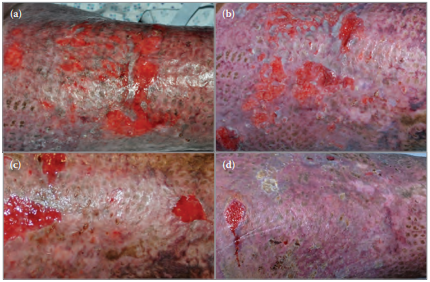
Figure 4. Mr X's thigh wounds during the course of treatment with Actilite.
Case Study 2
Mr Y is a 51-year-old man who sustained a full thickness burn to his chest and in the cleft of his buttocks (Figure 5a). How Mr Y sustained these burns is unknown; his suggestion that it was the result of friction against carpet during a seizure was not consistent with the clinical presentation.
The burn on his chest was initially treated with FLAMAZINE™ Cream (Smith & Nephew) but failed to progress to healing (Figure 5b) and Algivon Plus was initiated at day 58 following presentation. Algivon Plus is shown in situ in Figure 5c.
The improvement in Mr Y’s wound is shown at 31 and 44 days following initiation of treatment with Algivon Plus (Figures 5d–e, respectively), and by day 52, Mr Y’s wound had healed (Figures 5f).
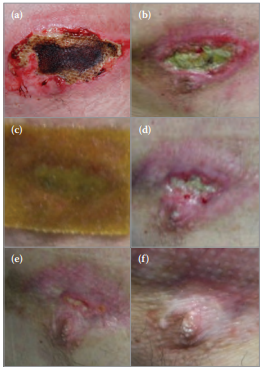
Figure 5. Mr Y's chest wounds during the course of treatment with Actilite.
Discussion and Conclusion
As can be seen from Figure 1, Algivon Plus appeared to have out-performed Actilite. However, the most important aspect was patient selection. A number of patients who had Algivon Plus applied had to have it removed, due to the pain from the honey, within 30 minutes. This happened more frequently with new burns, even those that were deep dermal / full thickness. With Actilite, no patients refused to have the dressing reapplied, but it seemed to perform better on patients with wound breakdown rather than newer burns.
There is a need to greater understand which wound types work better with which product. Patient acceptability of Actilite was higher with 100% of patients stating the product was acceptable. This was used on predominantly granulating wounds which were slow to heal. Chronicity in burn wounds is under investigated and recognised even within burn services. Consequently, products intended to promote healing in nonhealing wounds are often under used. Actilite seems to have a place in this wound group and perhaps will help to promote understanding of the need to manage nonhealing burn wounds in a different way.
Patients with Algivon Plus experienced pain at application and so only 75% of patients found the dressing acceptable. These wounds were largely sloughy or necrotic, so it is interesting that they experienced the pain. However, this might have been down to patient selection, and more experience with the dressing is needed to understand this phenomenon.
“The majority of staff felt confident in using the product and would recommend it for further use as it worked well and there seems to be a place for the honey range of products in managing burn wounds."
All patients in which full healing was achieved were followed up 3–4 weeks post-healing and to date none of them have developed hypertrophic or other abnormal scarring. This is potentially significant given that Van den Kerckhove et al (2001) suggest that the development of hypertrophic scars is one of the most common and frustrating problems after burn injury, due to both the functional and aesthetic consequences. Schmidt et al (2001) suggest that hypertrophic scars appear between 3–5 weeks after trauma and that usually if there is no evidence of hypertrophic scarring at this point then it is unlikely to develop in the future. There are a number of extrinsic factors that are suggested to be important in hypertrophic scar development such as infection, type of wound intervention used, and tension of the wound (Van den Kerckhove et al, 2001). It could be that, by controlling infection during the wound healing period with honey dressings, that this has a positive effect on the absence of hypertrophic scarring. This would need to be investigated more thoroughly, but shows some interesting results.
References
Advancis Medical (2008) Activon Tube, Activon Tulle, Algivon and Actilite: Antibacterial potency from Manuka honey. Wound Essentials 3: 165
Belcher J (2012) A review of medical-grade honey in wound care. Br J Nurs 21(15): S4–9
Wounds UK (2011) Best Practice Statement: The use of Topical Antiseptic/ Antimicrobial Agents in Wound Management (2nd edn). Wounds UK, London
DeSanti L (2005) Pathophysiology and current management of burn injury. Adv Skin Wound Care 18(6): 323–32
Jull AB, Rodgers A, Walker N (2008) Honey as a topical treatment for wounds. Cochrane Database Syst Rev (4): CD005083
Schmidt A, Gassmueller J, Hughes-Formella B, Bielfeldt S (2001) Treating hypertrophic scars for 12 or 24 hours with a self-adhesive hydroactive polyurethane dressing. J Wound Care 10(5): 149–53
Stephen-Haynes J, Callaghan R (2011) Properties of honey: its mode of action and clinical outcomes. Wounds UK 7(1): 50–7
Subrahmanyam M, Hemmady AR, Pawar SG (2003) Mulitidrugresistant Staphylococcus aureus isolated from infected burns sensitive to honey. Ann Burn Fire Disasters 16: 192–3
Van den Kerckhove E, Stappaerts K, Boeckx W et al (2001) Silicones in the rehabilitation of burns: a review and overview. Burns 27(3): 205–14
